
Browsing Toxins By Category
Displaying toxin 1676 - 1700 of 3678 in total
| T3DB ID | Name CAS Number | Formula Weight | Structure | Type | Mechanism of Toxicity |
|---|---|---|---|---|---|
| T3D1356 | Mercurol 12002-19-6 | C4H4Hg 252.660 g/mol | 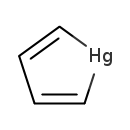 |
| High-affinity binding of the divalent mercuric ion to thiol or sulfhydryl groups of proteins is believed to be the major mechanism for the activity of mercury. Through...more Number of Targets: 50 |
| T3D4540 | Thiacloprid 111988-49-9 | C10H9ClN4S 252.723 g/mol | 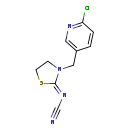 |
| Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxi...more Number of Targets: 5 |
| T3D1785 | Bromoform 75-25-2 | CHBr3 252.731 g/mol | 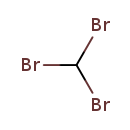 |
| Bromine is a powerful oxidizing agent and is able to release oxygen free radicals from the water in mucous membranes. These free radicals are also potent oxidizers and...more Number of Targets: 0 |
| T3D1606 | Silver hexafluorophosphate 26042-63-7 | AgF6P 252.832 g/mol | 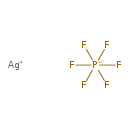 |
| Metallic silver is oxidized and may deposit in the tissues, causing arygria. The silver ion is known to inhibit glutathione peroxidase and NA+,K+-ATPase activity, disr...more Number of Targets: 17 |
| T3D2048 | 1,3-Dichlorodibenzo-p-dioxin 50585-39-2 | C12H6Cl2O2 253.081 g/mol | 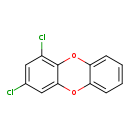 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2049 | 1,6-Dichlorodibenzo-p-dioxin 38178-38-0 | C12H6Cl2O2 253.081 g/mol | 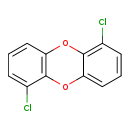 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2050 | 2,3-Dichlorodibenzo-p-dioxin 29446-15-9 | C12H6Cl2O2 253.081 g/mol | 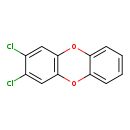 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2051 | 2,7-Dichlorodibenzo-p-dioxin 33857-26-0 | C12H6Cl2O2 253.081 g/mol | 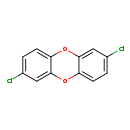 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2052 | 2,8-Dichlorodibenzo-p-dioxin 38964-22-6 | C12H6Cl2O2 253.081 g/mol | 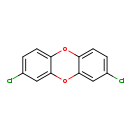 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2053 | 1,4-Dichlorodibenzo-p-dioxin 54536-19-5 | C12H6Cl2O2 253.081 g/mol | 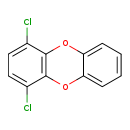 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2054 | 1,2-Dichlorodibenzo-p-dioxin 54536-18-4 | C12H6Cl2O2 253.081 g/mol | 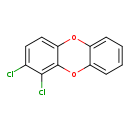 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2055 | 1,9-Dichlorodibenzo-p-dioxin 64560-13-0 | C12H6Cl2O2 253.081 g/mol | 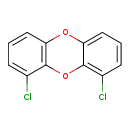 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2056 | 1,8-Dichlorodibenzo-p-dioxin 82291-27-8 | C12H6Cl2O2 253.081 g/mol | 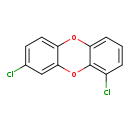 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2057 | 1,7-Dichlorodibenzo-p-dioxin 82291-26-7 | C12H6Cl2O2 253.081 g/mol | 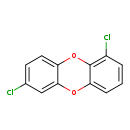 |
| CDDs cause their toxic effects by binding to the aryl hydrocarbon receptor and subsequently altering the trascription of certain genes. The affinity for the Ah recepto...more Number of Targets: 4 |
| T3D2329 | 7,8-Dichloro-2-dibenzofuranol 74423-77-1 | C12H6Cl2O2 253.081 g/mol | 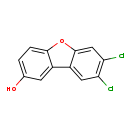 |
| Halogenated dibenzofurans (PCDFs and PBDFs) bind the aryl hydrocarbon receptor (AhR), which increases its ability to activate transcription in the XRE (xenobiotic reso...more Number of Targets: 2 |
| T3D3813 | Clopyralid-olamine 57754-85-5 | C8H10Cl2N2O3 253.083 g/mol | 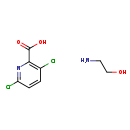 |
| Not Available Number of Targets: 10 |
| T3D0040 | 3,3'-Dichlorobenzidine 91-94-1 | C12H10Cl2N2 253.127 g/mol | 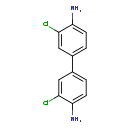 |
| 3,3’-Dichlorobenzidine's mechanism of toxicity derives mainly from the adduction of DNA by its metabolites. The formation of nitroso derivatives during metabolism, yie...more Number of Targets: 50 |
| T3D4162 | Neopterin 2009-64-5 | C9H11N5O4 253.215 g/mol | 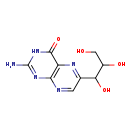 |
| Uremic toxins such as neopterin are actively transported into the kidneys via organic ion transporters (especially OAT3). Increased levels of uremic toxins can stimula...more Number of Targets: 3 |
| T3D0924 | Asulam, sodium salt 2302-17-2 | C8H10N2NaO4S 253.231 g/mol | 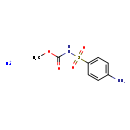 |
| Asulam, sodium salt is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylc...more Number of Targets: 2 |
| T3D1677 | Isobornyl thiocyanoacetate 115-31-1 | C13H19NO2S 253.360 g/mol |  |
| Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxi...more Number of Targets: 39 |
| T3D2862 | Tizanidine 51322-75-9 | C9H8ClN5S 253.711 g/mol | 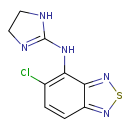 |
| Tizanidine reduces spasticity by increasing presynaptic inhibition of motor neurons through agonist action at a2-adrenergic receptor sites. Number of Targets: 4 |
| T3D1743 | Nitrogen tribromide 15162-90-0 | Br3N 253.719 g/mol | 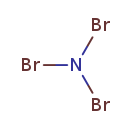 |
| Bromine is a powerful oxidizing agent and is able to release oxygen free radicals from the water in mucous membranes. These free radicals are also potent oxidizers and...more Number of Targets: 6 |
| T3D3596 | Iodine 7553-56-2 | I2 253.809 g/mol |  |
| Iodide inhibits adenylate cyclase in thyroid gland follicle cells and decreases the TSH-induced rise in intracellular cAMP. This results in decreased iodination of thy...more Number of Targets: 10 |
| T3D1902 | Tris(dimethylamino)antimony 7289-92-1 | C6H18N3Sb 253.990 g/mol | 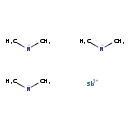 |
| The inhalation data suggests that the myocardium is a target of antimony toxicity. It is possible that antimony affects circulating glucose by interfering with enzymes...more Number of Targets: 20 |
| T3D1173 | Uranium carbide 12070-09-6 | CH4U 254.071 g/mol | 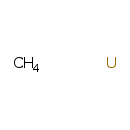 |
| Uranium is combined with either bicarbonate or a plasma protein in the blood but once in the kidney, it is released and forms complexes with phosphate ligands and prot...more Number of Targets: 1 |