
Browsing Toxins By Category
Displaying toxin 1501 - 1525 of 3678 in total
| T3DB ID | Name CAS Number | Formula Weight | Structure | Type | Mechanism of Toxicity |
|---|---|---|---|---|---|
| T3D0955 | Desmedipham 13684-56-5 | C16H16N2O4 300.309 g/mol | 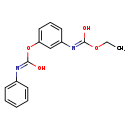 |
| Desmedipham is a cholinesterase or acetylcholinesterase (AChE) inhibitor. Carbamates form unstable complexes with chlolinesterases by carbamoylation of the active site...more Number of Targets: 4 |
| T3D0994 | Phenmedipham 13684-63-4 | C16H16N2O4 300.309 g/mol | 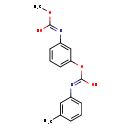 |
| Phenmedipham is a cholinesterase or acetylcholinesterase (AChE) inhibitor. Carbamates form unstable complexes with chlolinesterases by carbamoylation of the active sit...more Number of Targets: 5 |
| T3D0846 | Phellopterin 2543-94-4 | C17H16O5 300.306 g/mol | 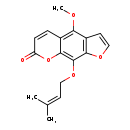 |
| The mechanism of action many furocoumarins is based on their ability to form photoadducts with DNA and other cellular components such as RNA, proteins, and several pro...more Number of Targets: 4 |
| T3D2804 | Chlordiazepoxide 58-25-3 | C16H14ClN3O 299.755 g/mol | 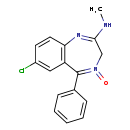 |
| Chlordiazepoxide binds to stereospecific benzodiazepine (BZD) binding sites on GABA (A) receptor complexes at several sites within the central nervous system, includin...more Number of Targets: 18 |
| T3D3621 | Cocoyl sarcosine 783292-49-9 | C17H33NO3 299.449 g/mol | 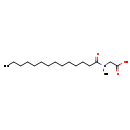 |
| While acyl sarcosines themselves are not toxic, they are nitrosating agents. Nitrosating agents may decompose and/or react to cause nitrosamine contamination. Nitrosam...more Number of Targets: 2 |
| T3D2941 | Hydrocodone 125-29-1 | C18H21NO3 299.364 g/mol | 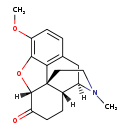 |
| Hydrocodone acts as a weak agonist at OP1, OP2, and OP3 opiate receptors within the central nervous system (CNS). Hydrocodone primarily affects OP3 receptors, which ar...more Number of Targets: 3 |
| T3D2749 | Codeine 76-57-3 | C18H21NO3 299.364 g/mol |  |
| Opiate receptors are coupled with G-protein receptors and function as both positive and negative regulators of synaptic transmission via G-proteins that activate effec...more Number of Targets: 5 |
| T3D4739 | Imipenem 74431-23-5 | C12H17N3O4S 299.346 g/mol | 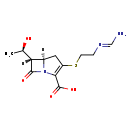 |
| Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria. This inhibition of cell wall synth...more Number of Targets: 0 |
| T3D2551 | Saxitoxin 35523-89-8 | C10H17N7O4 299.287 g/mol | 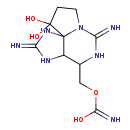 |
| Saxitoxin blocks voltage-gated sodium channels of nerve cells. (L1115) Number of Targets: 14 |
| T3D0878 | Antimony pentachloride 7647-18-9 | Cl5Sb 299.030 g/mol | 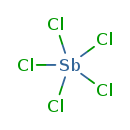 |
| The inhalation data suggest that the myocardium is a target of antimony toxicity. It is possible that antimony affects circulating glucose by interfering with enzymes ...more Number of Targets: 20 |
| T3D1861 | Antimony(III) isopropoxide 18770-47-3 | C9H21O3Sb 299.020 g/mol | 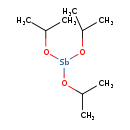 |
| The inhalation data suggest that the myocardium is a target of antimony toxicity. It is possible that antimony affects circulating glucose by interfering with enzymes ...more Number of Targets: 20 |
| T3D1905 | Antimony acetate 3643-76-3 | C6H9O6Sb 298.890 g/mol | 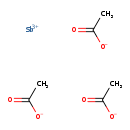 |
| The inhalation data suggests that the myocardium is a target of antimony toxicity. It is possible that antimony affects circulating glucose by interfering with enzymes...more Number of Targets: 20 |
| T3D1350 | Methylmercuric dicyanamide 502-39-6 | C3H6HgN4 298.700 g/mol | 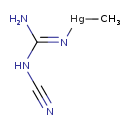 |
| High-affinity binding of the divalent mercuric ion to thiol or sulfhydryl groups of proteins is believed to be the major mechanism for the activity of mercury. Through...more Number of Targets: 88 |
| T3D4745 | Norethindrone 68-22-4 | C20H26O2 298.419 g/mol | 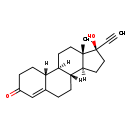 |
| Progestins diffuse freely into target cells and bind to the progesterone receptor. Target cells include the female reproductive tract, the mammary gland, the hypothala...more Number of Targets: 6 |
| T3D3967 | Enterolactone 76543-15-2 | C18H18O4 298.333 g/mol | 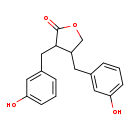 |
| Not Available Number of Targets: 3 |
| T3D3714 | Toxin T2 tetrol 34114-99-3 | C15H22O6 298.332 g/mol | 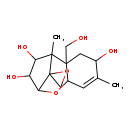 |
| Unlike many other mycotoxins, trichothecenes do not require metabolic activation to exert their biological activity, instead directly reacting with cellular components...more Number of Targets: 2 |
| T3D3103 | Fumarin 117-52-2 | C17H14O5 298.290 g/mol | 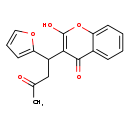 |
| Fumarin inhibits the enzyme Vitamin K epoxide reductase. This enzyme is needed for the reconstitution of the vitamin K in its cycle from vitamin K-epoxide, and so fuma...more Number of Targets: 1 |
| T3D4686 | Cisplatin 15663-27-1 | Cl2H4N2Pt 298.035 g/mol | 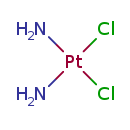 |
| Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases, resulting in the DNA being fragmented by repair enzymes in their atte...more Number of Targets: 1 |
| T3D4781 | Hydrochlorothiazide 58-93-5 | C7H8ClN3O4S2 297.739 g/mol | 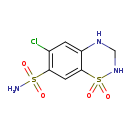 |
| Hydrochlorothiazide, a thiazide diuretic, inhibits water reabsorption in the nephron by inhibiting the sodium-chloride symporter (SLC12A3) in the distal convoluted tub...more Number of Targets: 9 |
| T3D4939 | Tridemorph 81412-43-3 | C19H39NO 297.519 g/mol | 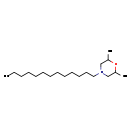 |
| Not Available Number of Targets: 1 |
| T3D3923 | Spiroxamine 118134-30-8 | C18H35NO2 297.476 g/mol | 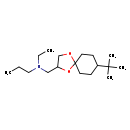 |
| Not Available Number of Targets: 20 |
| T3D2805 | Duloxetine 136434-34-9 | C18H19NOS 297.415 g/mol | 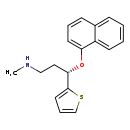 |
| Duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a less potent inhibitor of dopamine reuptake. Duloxetine has no significant affi...more Number of Targets: 3 |
| T3D1425 | Potassium tetraperoxochromate(V) 12331-76-9 | CrK3O8 297.286 g/mol | 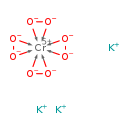 |
| Chromium has been shown to induce carcinogenesis by overstimulating cellular regulatory pathways and increasing peroxide levels by activating certain mitogen-activated...more Number of Targets: 4 |
| T3D3860 | Enilconazole 35554-44-0 | C14H14Cl2N2O 297.180 g/mol | 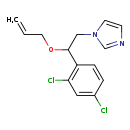 |
| Not Available Number of Targets: 36 |
| T3D1095 | Barium bromide 10553-31-8 | BaBr2 297.135 g/mol | 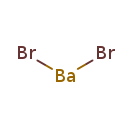 |
| Barium is a competitive potassium channel antagonist that blocks the passive efflux of intracellular potassium, resulting in a shift of potassium from extracellular to...more Number of Targets: 27 |