
Browsing Toxins By Category
Displaying toxin 2001 - 2025 of 3678 in total
| T3DB ID | Name CAS Number | Formula Weight | Structure | Type | Mechanism of Toxicity |
|---|---|---|---|---|---|
| T3D3096 | D-Hyoscyamine 13269-35-7 | C17H23NO3 289.369 g/mol | 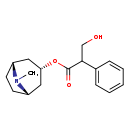 |
| Hyoscyamine is an anticholinergic, specifically an antimuscarinic, and works by blocking the action of acetylcholine at parasympathetic sites in smooth muscle, secreto...more Number of Targets: 6 |
| T3D4356 | D-Glucose 50-99-7 | C6H12O6 180.156 g/mol | 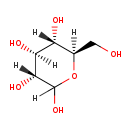 |
| Very high serum levels of glucose are found in untreated diabetic (type I or type II) patients. Glucose in chronic excess causes toxic effects on the structure and fun...more Number of Targets: 33 |
| T3D1157 | D-Gluconic acid Mn(II) salt 6485-39-8 | C6H12O7 196.155 g/mol | 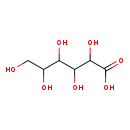 |
| Manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor functions. It primarily targets the central nervous system, particu...more Number of Targets: 5 |
| T3D4345 | D-Fructose 53188-23-1 | C6H12O6 180.156 g/mol | 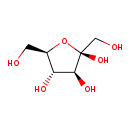 |
| Fructose is distinct from other sugars in its ability to cause intracellular ATP depletion, nucleotide turnover, and the generation of uric acid. Uric acid is generate...more Number of Targets: 5 |
| T3D4251 | D-2-Hydroxyglutaric acid 2889-31-8 | C5H8O5 148.114 g/mol | 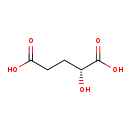 |
| 2-hydroxyglutarate is an oncometabolite. It is a competitive inhibitor of multiple α-ketoglutarate-dependent dioxygenases, including histone demethylases and the TET f...more Number of Targets: 16 |
| T3D3681 | Cytochalasin J 56144-22-0 | C28H35NO5 465.581 g/mol | 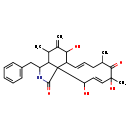 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D3680 | Cytochalasin H 53760-19-3 | C30H39NO5 493.634 g/mol | 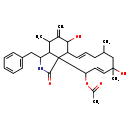 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D3679 | Cytochalasin E 36011-19-5 | C27H33NO7 483.553 g/mol | 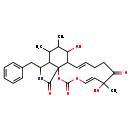 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D3678 | Cytochalasin D 22144-77-0 | C30H37NO6 507.618 g/mol | 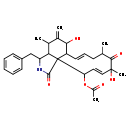 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D3677 | Cytochalasin C 22144-76-9 | C30H37NO6 507.618 g/mol |  |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D3667 | Cytochalasin B 14930-96-2 | C29H37NO5 479.608 g/mol | 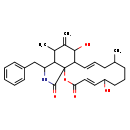 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 7 |
| T3D3676 | Cytochalasin A 14110-64-6 | C29H35NO5 477.592 g/mol | 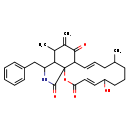 |
| Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monome...more Number of Targets: 6 |
| T3D4978 | Cytarabine 147-94-4 | C9H13N3O5 243.217 g/mol | 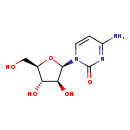 |
| Cytarabine acts through direct DNA damage and incorporation into DNA. Cytarabine is cytotoxic to a wide variety of proliferating mammalian cells in culture. It exhibit...more Number of Targets: 3 |
| T3D4493 | Cyromazine 66215-27-8 | C6H10N6 166.184 g/mol | 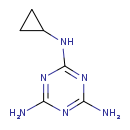 |
| Not Available Number of Targets: 3 |
| T3D4747 | Cyproterone acetate 427-51-0 | C24H29ClO4 416.938 g/mol | 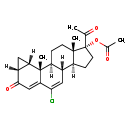 |
| The direct antiandrogenic effect of cyproterone is blockage of the binding of dihydrotestosterone to the specific receptors in the prostatic carcinoma cell. In additio...more Number of Targets: 4 |
| T3D2790 | Cyproheptadine 129-03-3 | C21H21N 287.398 g/mol |  |
| Cyproheptadine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the ...more Number of Targets: 17 |
| T3D3820 | Cyprodinil 121552-61-2 | C14H15N3 225.289 g/mol | 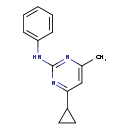 |
| Not Available Number of Targets: 18 |
| T3D4492 | Cyproconazole 94361-06-5 | C15H18ClN3O 291.776 g/mol | 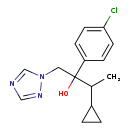 |
| Not Available Number of Targets: 14 |
| T3D1033 | Cyphenothrin 39515-40-7 | C24H25NO3 375.460 g/mol | 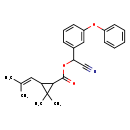 |
| Both type I and type II pyrethroids exert their effect by prolonging the open phase of the sodium channel gates when a nerve cell is excited. They appear to bind to th...more Number of Targets: 19 |
| T3D1034 | Cypermethrin 52315-07-8 | C22H19Cl2NO3 416.297 g/mol | 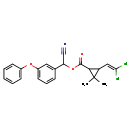 |
| Both type I and type II pyrethroids exert their effect by prolonging the open phase of the sodium channel gates when a nerve cell is excited. They appear to bind to th...more Number of Targets: 40 |
| T3D0952 | Cypendazole 28559-00-4 | C16H19N5O3 329.354 g/mol | 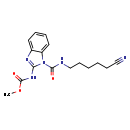 |
| Cypendazole is a cholinesterase or acetylcholinesterase (AChE) inhibitor. Carbamates form unstable complexes with chlolinesterases by carbamoylation of the active site...more Number of Targets: 2 |
| T3D3819 | Cymoxanil 57966-95-7 | C7H10N4O3 198.179 g/mol | 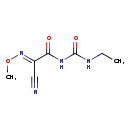 |
| Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxi...more Number of Targets: 5 |
| T3D4232 | Cylindrospermopsin 143545-90-8 | C15H21N5O7S 415.422 g/mol | 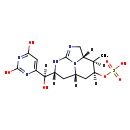 |
| Not Available Number of Targets: 1 |
| T3D1032 | Cyhalothrin 68085-85-8 | C23H19ClF3NO3 449.850 g/mol | 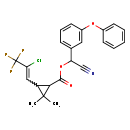 |
| Both type I and type II pyrethroids exert their effect by prolonging the open phase of the sodium channel gates when a nerve cell is excited. They appear to bind to th...more Number of Targets: 20 |
| T3D3818 | Cyhalofop-butyl 122008-85-9 | C20H20FNO4 357.376 g/mol | 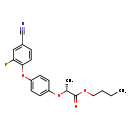 |
| Organic nitriles decompose into cyanide ions both in vivo and in vitro. Consequently the primary mechanism of toxicity for organic nitriles is their production of toxi...more Number of Targets: 4 |